Smoothly does it: Structural insights into an unusual G-protein coupled receptor
Publication date: 2016
A collaborative project between the Newstead and Sansom groups in the Department of Biochemistry and Christian Siebold’s group in the Division of Structural Biology has led to the first high resolution structure of a full length G-Protein Coupled Receptor called Smoothened.
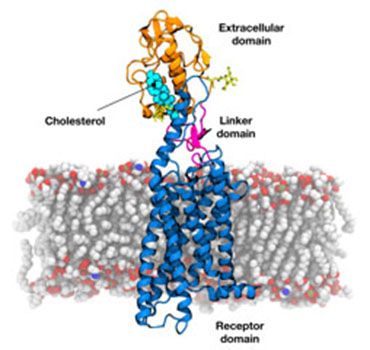 Crystal Structure of human Smoothened receptor embedded in a lipid bilayer.
Crystal Structure of human Smoothened receptor embedded in a lipid bilayer.(Credit: George Hedger)
The research published in Nature (1) reveals for the first time how the extra-cellular ligand binding cysteine-rich domain docks above the canonical hepta helical receptor domain.
The structure also reveals many new and exciting insights into the regulation of this key developmental receptor. Most notable was the discovery that cholesterol binds to the extra-cellular domain. In the absence of cholesterol, the cysteine-rich domain possesses a degree of conformational flexibility, while cholesterol binding stabilizes this domain via key interactions. Experiments carried out in a mouse knock out model by Rajat Rohatgi in the Department of Biochemistry and Medicine, Stanford, USA show that cholesterol binding has an important and unexpected role in switching off the receptor during development.
The current study also reports the structure of Smoothened bound to the anti- cancer drug vismodegib, which shows that drug and cholesterol bind to different sites. These new insights may lead to new routes for drug development.
Professor Newstead comments that this breakthrough was made possible through the generous support of the EPA Cephalosporin fund, which allowed the purchase of the Lipid Cubic Phase crystallization robot for the Crystallisation Facility within the Department of Biochemistry and that has allowed our continued success in membrane protein structural biology.
(1) Structural basis of Smoothened regulation by its extracellular domains. Eamon F.X. Byrne, Ria Sircar, Paul Miller, George Hedger, Sigrid Nachtergaele, Giovanni Luchetti, Mark S. P. Sansom, Simon Newstead, Rajat Rohatgi, Christian Siebold. Nature, 2016 Jul 20;535(7613):517-522.