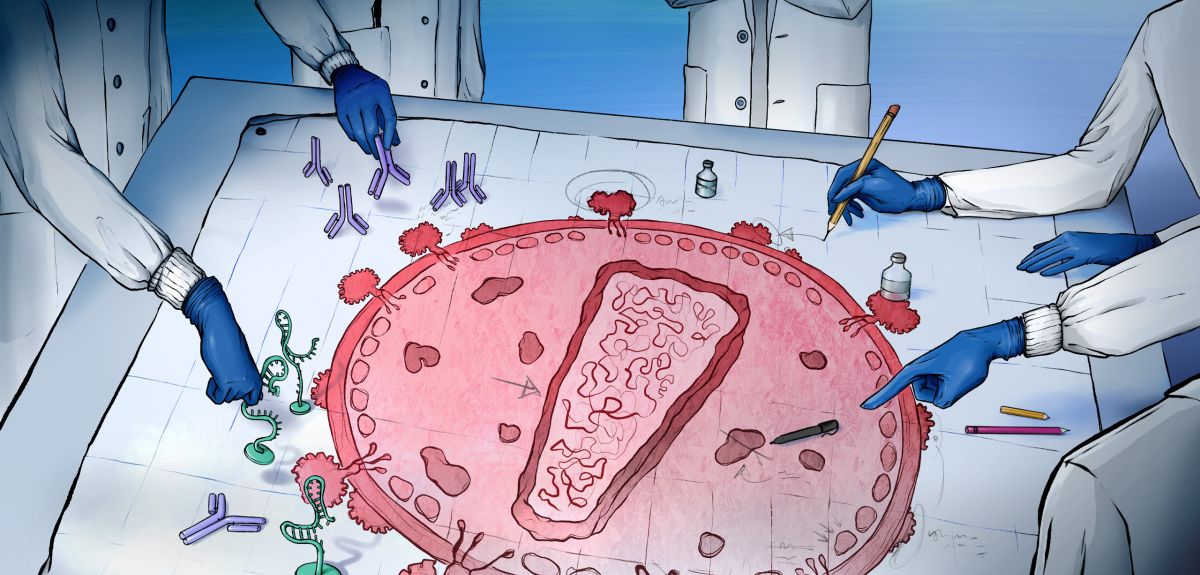
EAVI2020: The Quest for an HIV Vaccine
In this long read published to coincide with International AIDS Day, we explore how an international collaboration – of which the University of Oxford is a key partner – has boosted HIV vaccine research. We thank our partners at Imperial College London for allowing us to reproduce and abridge this article.
Since COVID-19 reared its head in December 2019, the global scientific community has developed several effective vaccines against the virus. The US Food and Drug Administration (FDA) approved the Pfizer-BioNTech vaccine in December 2020, just one year after COVID-19 was first detected. Around the same time, the UK government also approved a vaccine developed by the University of Oxford in partnership with AstraZeneca.
HIV has been around for decades, and hundreds of thousands of people around the world die every year from the disease, yet no effective vaccine exists.
‘Without a vaccine, we will not be able to control the AIDS epidemic,’ said Tomáš Hanke, Professor of Vaccine Immunology at the Jenner Institute, Nuffield Department of Medicine, University of Oxford.
‘We’ve been trying to develop a vaccine for HIV for over 30 years as a field,’ said Professor Robin Shattock, Head of Mucosal Infection and Immunity within the Faculty of Medicine at Imperial College London.
‘It still remains one of the biggest biological challenges of a generation.’
Introducing EAVI2020
International problems require international solutions.
If the past few years have taught us anything, it’s that science is at its best when researchers come together to work towards a common goal.
This was the basis of the European AIDS Vaccine Initiative (EAVI2020). Launched in 2015, and concluding in December 2022, EAVI2020 was a 23 million Euro project funded by the European Commission. The project brought together 22 teams with different expertise and ideas from across Europe, with additional partners in Australia and Canada, to further progress towards an HIV vaccine.
‘There was a great sense of excitement,” recalled Professor Shattock, who led the project. ‘We were bringing together teams from all across Europe at a critical time in HIV research where scientific progress had revealed unprecedented insight into the nature of protective antibodies and anti-viral cellular responses that are needed for an effective vaccine.’
‘Scientists at the University of Oxford, including Professor Sir Andrew McMichael, Professor Quentin Sattentau, Max Crispin and I had been working on vaccine development for decades and were delighted to join the EAVI2020 effort.’ says Professor Hanke.
The EAVI2020 consortium members consisted of molecular biologists, immunologists, virologists, biotechnologists and clinicians. Collectively, they provided the breadth of expertise required to take the latest discoveries in the lab and rapidly advance them through preclinical testing and manufacture, and into early-stage human trials.
Where to begin?
You may wonder how a project would even start to approach a problem like developing an HIV vaccine.
Firstly, we need to understand that there are two main kinds of possible HIV vaccine:
- Preventative (or prophylactic): the classic idea of a vaccine, where receiving the vaccine prevents you from contracting the infection and/or developing disease.
- Therapeutic (or curative): a vaccine that would be given to someone with an existing infection, aiming to boost the immune system so that the body can slow or eradicate the virus.
Both approaches would need to induce a strong immune response in order to fight off HIV. There are two arms of the body’s immune system that a vaccine can focus on inducing – ‘humoral’ and ‘cellular’.
The humoral immune response relies on the vaccine activating B cells, a type of immune cell, to produce antibodies that bind to the virus and prevent it from infecting your cells.
The cellular immune response is where T cells, another type of immune cell, seek out and kill infected cells.
‘Tackling both of those approaches was important because in our minds we knew we'd have to bring together both arms of the adaptive immune responses to develop an effective vaccine,’ said Professor Shattock.
Knowing this is all well and good, but actually inducing these immune responses against HIV is where things get tricky.
Chasing a moving target
Viruses are known to mutate – everyone will recall that there have been several well-documented variants of COVID-19. But HIV operates on another scale, mutating incredibly quickly to produce new strains.
Not only are there many, many strains of HIV globally, but the virus is also variable even within a single infected individual.
‘HIV variability is daunting, but there is nothing that human ingenuity wouldn’t want to crack,’ notes Professor Hanke. ‘However, this nut has been extremely hard.’
 Tomáš Hanke (far right) and his team - courtesy photo
Tomáš Hanke (far right) and his team - courtesy photoThe continuously changing nature of HIV means that the body’s immune system finds it very difficult to produce antibodies that can fight off the virus, as it can rapidly change itself so that those antibodies are no longer effective.
Professor Rogier Sanders from Universitair Medische Centra in Amsterdam led EAVI2020’s charge in developing approaches to stimulate the production of effective antibodies against HIV, bringing with him the results of his previous research.
‘I had been working on HIV envelope vaccines for a long time,’ says Professor Sanders.
‘We had somewhat of a breakthrough just before EAVI2020 started with the development of what we call “native-like envelope trimers”. The envelope protein is on the outside of the virus, but it’s unstable and falls apart easily when you try to put it into a vaccine. My team managed to stabilise it in such a way that it can be expressed in a vaccine candidate without worrying that it’ll fall apart.’
Professor Sanders and his team took around 20 years to develop just one example of this kind of vaccine, but managed to create eight new versions of this design and entering them in clinical studies over the course of EAVI2020. These were some of the first clinical trials to be looking at this kind of vaccine.
Whilst Professor Sanders focussed his efforts on developing a vaccine to induce the humoral arm of the immune system, Professors Hanke and Christian Brander from IrsiCaixa, Barcelona, took on the challenge of inducing the body’s cellular immune protection.
‘The main challenge is the same – the variability of the virus,’ said Professor Hanke. ‘But the problems associated with tackling this variability are different between inducing broadly neutralising antibodies and inducing protective killer T cells.’
The vaccine candidate designed by Professor Hanke tackles this variability by focusing on the most functionally conserved regions of the HIV virus, which are the parts that change the least and are common to most global variants as well.
Professor Brander notes that a possible solution comes from some people living with HIV themselves.
‘We know that there are some people with good control of the virus on their own without having to take antiretrovirals. Everyone who becomes infected with the virus makes a roaring T cell response, which is, however, mostly inefficient. In a previous study, we found specific regions of HIV, responses to which were associated with low virus load, so we combined them into a singular construct to test in a vaccine.’
Test and analysis
Developing vaccine candidates is just one step towards an effective HIV vaccine. Each of these candidates must go through rigorous testing and analysis for both safety and effectiveness.
Candidate vaccines had to leap various hurdles as part of the EAVI2020 project, being tested first in rabbits, then macaques (a type of monkey), before being trialled in humans.
‘A key part of EAVI2020 involved finding which animal models are the most predictive [of results in humans], in order to reduce the number of animals that have to be tested on in future projects,’ said Dr Nathalie Dereuddre-Bosquet, a specialist in non-human primate immunology and on preclinical models for vaccine development from the Infectious Disease Models and Innovative Therapies (IDMIT) Infrastructure within the Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA) in Paris.
‘We only use macaques in late-stage preclinical development of vaccines for the most promising candidates. It’s important to test vaccines in macaques as their immune systems and physiology are really much closer to humans. For instance, non-human-primate models are crucial to identify and understand interactions between B-cell and T-cell vaccines.’
Only vaccines that overcome these hurdles can then be trialled in humans, initially in a small group to measure safety, before going onto larger groups to test how well the vaccine actually works.
A key player involved at all stages of this process is Dr Gabriella Scarlatti from Ospedale San Raffaele in Milan, whose team analyses biological material, such as blood samples, to ascertain whether the vaccine is good enough to progress.
‘We had the task of testing the samples to see if there is a change between before and after immunisation,’ said Dr Scarlatti.
‘For example, we want to see if vaccines have increased capacity to induce neutralising antibodies. Neutralizing antibodies are the Holy Grail of HIV vaccinology as they not only bind to the virus but also prevent it from entering and infecting the cell.’
Another aspect that Dr Scarlatti’s team looked for were broadly reactive antibodies i.e. antibodies that could neutralise multiple strains of HIV. This is vital so that any future vaccine can deliver as much protection as possible.
A two-pronged approach
EAVI2020 ran eight clinical studies in humans during the course of its project, most of which have finished, but some are still underway at the time of writing.
A key trial for Professor Brander is one that is understatedly named BCN03. This is testing a vaccine that brings together his work on inducing the cellular immune response with Professor Sanders’ work on inducing broadly neutralizing antibodies.
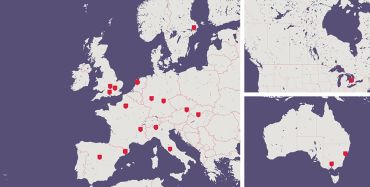 A map depicting the various EAVI sites - courtesy photo
A map depicting the various EAVI sites - courtesy photo‘BCN03 is the first therapeutic vaccine trial that combines T and B cell immunogens,’ said Professor Brander. ‘In EAVI2020, we always wanted to produce B cell and T cell immunogens and to test them individually, but we managed to combine them together, which was originally planned to be achieved after the project.’
Currently, in the therapeutic setting, the focus is on inducing the cellular immune responses – teaching T cells to kill cells producing HIV. They’re now layering in inducing humoral immunity as well.
This concept is new, and in Professor Brander’s opinion, very promising.
‘For me this is really one of the culminations of the programme, especially because it’s ahead of what we planned to achieve during EAVI2020.
‘Getting a better appreciation for the importance of having the humoral immune response and the cellular immune response coming together, and studying their interactions, is vital for both the therapeutic and the preventative setting.’
COVID-19: disruption and innovation
An unexpected spanner in the works came in the form of the COVID-19 pandemic.
‘When the COVID-19 pandemic took off, there were many restrictions across Europe and the EAVI2020 sites,” said Professor Shattock.
‘Many EAVI2020 laboratories closed or were repurposed for COVID-19 projects, affecting our HIV projects and clinical programmes.
‘Many of the consortium members also contributed either on the frontlines or into vaccine development for COVID-19.’
Professor Shattock himself led Imperial College London’s COVID-19 vaccine development programme and played a large role in the public awareness and understanding of vaccines in the UK.
Despite the impact of the pandemic on both the project and the world as a whole, there was a silver lining to the dark COVID cloud: the fantastic success of mRNA-based vaccines, which includes the Pfizer-BioNTech vaccine.
mRNA vaccines use a molecule called messenger RNA, which the body creates when expressing genes and acts as a blueprint for a protein molecule. mRNA vaccines use a piece of mRNA that corresponds to a protein from the virus it’s looking to target. The body’s cells then make the protein, which the immune system can react to and then remember if the body gets infected with the real virus.
Many of the vaccines that EAVI2020 had been developing up to this point were more complex and expensive to make compared to mRNA vaccines. BioNTech were one of the EAVI2020 partners looking at mRNA vaccines for HIV.
As the project managed to get up and going again, the team invested more effort into mRNA approaches for HIV as it meant they could create and test more vaccine candidates more quickly.
‘We managed to design and make brand new HIV vaccine candidates and tested them in the clinic in a matter of five years, which is really incredible, especially for the protein vaccines. Now with RNA, things will accelerate even further,’ said Professor Sanders.
More of the mRNA-based candidates have now been moved into pre-clinical studies, which Professor Shattock notes is already producing promising data that could be important for HIV vaccine development moving beyond EAVI2020 as the project comes to an end.
‘Also, T cell vaccines vectored by mRNA are in the pipeline for human testing,’ says Professor Hanke.
A success of collaboration
‘The greatest achievement [of EAVI2020] has been the success in bringing a large number of new vaccine candidates into clinical trials,’ said Professor Shattock.
‘For people who don’t inhabit this space, we have managed to produce more vaccines, test more vaccines, than any other programme of a similar scale, or even some funded to a much larger extent.’
‘Many thanks to Robin for his excellent coordination and leadership,’ notes Professor Hanke.
Looking to the future
Over the seven years of the project, EAVI2020 managed to develop vaccine candidates to stimulate both the humoral and cellular immune responses and was able to run many animal and human trials to test their safety and effectiveness in both preventative and therapeutic settings.
‘The impressive work of EAVI2020, the continuous development and improvement of vaccine candidates, means there is an enormous amount of data and material produced in the studies that can be analysed further,’ said Dr Scarlatti.
Several of the animal and human trials initiated by EAVI2020 are still ongoing, with one now being launched as the project comes to a close that will be run by Dr Scarlatti in Milan to test a new therapeutic T cell vaccine candidate designed by Professor Hanke.
The many data produced by EAVI2020, and the data still to come, are vital to making progress towards an effective HIV vaccine.
‘We’re already looking for the legacy of EAVI2020,’ said Professor Shattock.
‘We are collaborating with other groups to share some of these very sophisticated vaccines that we’ve developed that can be used in combination with approaches developed in other parts of the world. We are looking to see if any of these candidates merit additional development in their own right, or whether we should be looking at combinations.’
However, the next big challenge is finding the funding for this work.
‘The HIV vaccine field is beginning to struggle for recognition and further funding because of a false feeling that HIV has been solved by antiretroviral treatment,’ said Professor Hanke.
‘Treatment has been transformative, but it only buys time for the development of an effective vaccine, which will likely be a key part of any package to truly end the HIV epidemic.’
Whilst the future can never be certain, everyone who has contributed to EAVI2020 can be proud of the work they have done to progress the global effort to eradicate HIV, and they have shown the power of collaboration in making these strides.
‘It’s been an enormous privilege to work with such a dedicated and talented team,’ said Professor Shattock.
‘We’re challenged by the future, but challenge drives innovation. We’re well set to build on the legacy of what’s been achieved by EAVI2020.’
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 681137. The sole responsibility for the content of this project lies with the authors. It does not necessarily reflect the opinion of the European Union. The European Commission is not responsible for any use that may be made of the information contained therein.
Imperial College London wrote the original and unabridged version of this article.
 Statins do not cause the majority of side effects listed in package leaflets
Statins do not cause the majority of side effects listed in package leaflets
 Activism proves a stimulating topic at Sheldonian Series event
Activism proves a stimulating topic at Sheldonian Series event
 New Oxford-led initiative launches to train future leaders in transformative technologies for pharmaceutical research
New Oxford-led initiative launches to train future leaders in transformative technologies for pharmaceutical research