Features
A major exhibition on the 18th century poet and artist William Blake opens at the Ashmolean Museum today, featuring his illuminated books and manuscripts as well as a recreation of his studio.
Blake had little formal education and was isolated from the literary circles of his day. But today he is regarded as one of the major poets of his time: the famous opening line 'Tyger, Tyger, burning bright' is known to schoolchildren across the English-speaking world. Arts Blog explores how his reputation changed.
'Certainly, Blake was better known in his own time as an engraver. That was how he made his living,' said Professor Nicholas Halmi of the English Faculty at Oxford University. 'He wasn't regarded as a 'canonical' poet until after the Second World War.'
Blake was born in 1757 and apprenticed at 15 to a commercial engraver. 'He produced engravings of antiquarian objects - Greek urns and other artefacts - before going to work for a publisher,' said Professor Halmi. 'His poetry was known to a few people, including Wordsworth and Coleridge, although in fact Wordsworth thought he was mad.'
Blake's political and religious views were radical, in some respects even by 21st century standards, and these may have barred him from mainstream popularity, particularly at a time when Britain and France were still at war. 'Blake had a sense of a poet as a visionary or prophetic figure,' said Professor Halmi. 'Someone who had insight into society from the outside, and insight into the spiritual nature of man.
'He was strongly opposed to slavery and 'mental tyranny' - which for him included organised religion. He considered himself a Christian, and Christian themes are apparent in his works, but he hated what he referred to as the 'mind-forged manacles' of the Church. He believed they were not grounded in truth, and in fact kept people from perceiving the truth as he understood it, whereby a spark of divinity was present in all of humanity.'
Blake's unorthodox methods of publication present a more practical reason for the relative obscurity of his work. Although he did publish one conventionally printed book of poems, he moved on to produce small engraved editions of poetry. Approximately ten copies of each work would be printed, then coloured in watercolours by Blake's wife, Catherine. Blake then tried to sell these individually.
'Blake devised a kind of personal mythological system in these works,' said Professor Halmi. 'He drew on Norse and Biblical figures as well as his own private symbolic figures.'
Blake is still an anomalous figure among the "Big Six" male romantic poets, with Byron, Shelley, Wordsworth, Coleridge and Keats making up the remaining five. 'Blake had no formal schooling, nor was he immersed in the classical literary tradition, although he knew the Bible and English poetry very well,' said Professor Halmi. 'He had to work for a living, he had limited contact with literary circles, and he was not publishing his work conventionally.
'What is also unusual is his level of collaboration with his wife. We often think of the poet as a solitary genius, but printing is collaborative work. Blake had very forward-thinking views about men and women, and he and Catherine were together until death. When Blake was on his deathbed, it's said his last wish was to make a drawing of her.'
Blake's work remained a minority interest through the 19th century, but began to attract more attention into the 20th. Professor Halmi said: 'Later critics have either made use of, or resisted, the idea that Blake is a very obscure poet who has to be decoded or interpreted.
'Northrop Frye, a Canadian critic who tried to demystify Blake, believed that the work can be read as a coherent whole, with the engraved works at its centre. His study Fearful Symmetry, published in 1947, was largely responsible for bringing Blake into the canon. He said quite explicitly that we should think not of Blake as mad, but of the times we live in as mad. For Frye, Blake could offer some sanity to the post-war world.'
William Blake: Apprentice and Master opens on Thursday 4th December 2014 and runs until Sunday 1st March 2015.
A sweet-smelling chemical marker in the breath of children with type 1 diabetes could enable a handheld device to 'sniff out' the condition before a child becomes seriously ill.
That's according to news from the IOP about research reported in their Journal of Breath Research that was co-authored by Professor Gus Hancock of Oxford University's Department of Chemistry.
Professor Hancock is currently working with Oxford spin-out firm Oxford Medical Diagnostics to develop a prototype device, so I asked him about how this technology was progressing and how such a device might eventually be used…
OxSciBlog: Why is diagnosing type 1 diabetes early so important?
Gus Hancock: Untreated type 1 diabetes will result in the build-up of chemicals called ketones in the blood stream to levels which are well above normal values, and this can lead to a serious condition called diabetic ketoacidosis (DKA), when the blood becomes too acidic. This condition is the main cause of death in children with diabetes and can cause long-term problems. However, diabetes is fortunately quite straightforward to treat if caught early before ketoacidosis occurs and the child is well.
OSB: What link did you find between acetone in the breath and blood ketones?
GH: In this study we tested 113 children and young adults with type 1 diabetes for breath gases and blood constituents. The patients were all attending a regular clinic in the Oxford Children’s Hospital, and they all had their diabetes well controlled with insulin therapy.
We measured the concentration of a specific blood ketone which is used to determine the onset of DKA, and found that the values (which were all well below DKA levels) showed a strong correlation with the concentration of acetone on the breath - the higher values of the ketone were associated with higher levels of acetone. Chemically this is not a surprise it just needed to be proven in this cohort of patients.
OSB: What challenges are involved in developing a handheld device that can diagnose new diabetes from a child's breath?
GH: Let's answer two questions here. First, there are now no scientific challenges to be overcome to build a handheld device to measure acetone in breath of children or adults. The work at the Oxford Children's Hospital used a detection method called reactive ion mass spectrometry, which essentially identifies the molecules in breath by weighing them – not straightforward when there are up to 1000 detectable chemicals in breath. The instrument is the size of a washing machine and costs a quarter of a million pounds.
Our spin-out company, Oxford Medical Diagnostics, out at Begbroke Science Park, has now developed an optical technique to home in to the detection of the single most important diagnostic molecule, acetone. The technique is specific to acetone (ie it's not interfered with by the other 999 constituents) and is sensitive enough for medical needs. The prototype that we have built is the size of a shoe box, and professional designers have now reduced that to a hand held version which is shortly going into production. A single breath is processed presently in a couple of minutes, and that will decrease as we improve the engineering.
So, what would this be used for? People with type I diabetes are encouraged to test for ketones when they are not feeling well or have high blood glucose levels. This is because more insulin is needed if a child is ill, and if the insulin given is not enough, then ketones start to develop. This testing of ketone levels has to be done on a finger-prick blood test. We believe that a simple non-invasive breath test will be more acceptable (no blood required), and will be cost effective.
First, we have to prove that blood ketones and breath acetone track each other as DKA develops. We expect this: we have determined such tracking in non-diabetes volunteers when we induce mild ketoacidosis simply through diet and exercise. We envisage that the instrument will be used at home and could advise the patient whether or not ketone levels are increasing, so a decision can be made as to what should be done and whether or not hospital attendance is necessary.
Will it be used for diagnosis of new diabetes? Here we can certainly say that, if as expected, we find the tracking of blood ketones and breath acetone, it could act as a screening tool: high acetone levels will indicate that further tests are necessary. It will not immediately replace the gold standard of blood testing, but these are very early days, and we need clinical acceptance before we can make a sweeping statement about diagnosis.
OSB: What further research is needed in this area?
GH: We need more clinical data on people of all ages with type 1 diabetes, and are planning this in collaboration with the Oxford Hospitals Trust. We plan to give the handheld device to individuals so they can do frequent breath tests at home, particularly if they are feeling unwell, and we will also test patients who are admitted to hospital with DKA. For clinical use the device will need to pass a number of regulatory hurdles. And of course we are working on the handheld detection of other molecules which may be of importance in disease detection and management.
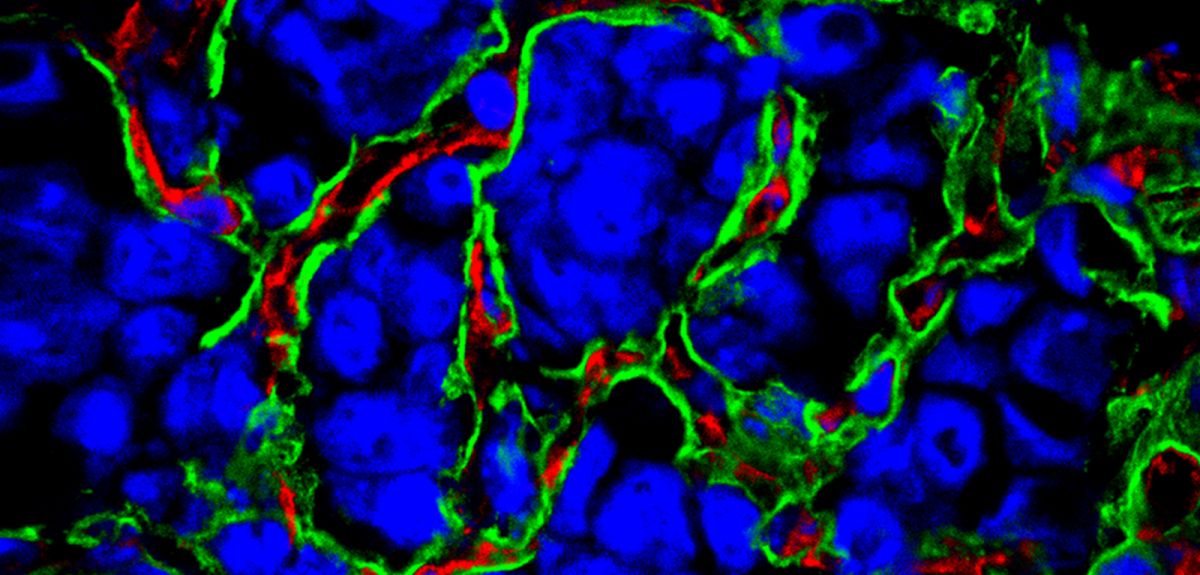
Cancer requires a blood supply to deliver the nutrients and oxygen it needs to grow and survive. It had been thought that tumours create the blood supply they need by stimulating the formation of new blood vessels, a process called angiogenesis.
But this no longer appears to be the only process going on. Some tumours seem to acquire their blood supply by taking over existing blood vessels, co-opting them for their cancerous growth.
Oxford Science Blog caught up with histopathologist Professor Francesco Pezella of the Radcliffe Department of Medicine at the University of Oxford, who has been establishing the role of blood vessel co-option in lung cancer. His group is now seeking to understand the process and says it could offer new avenues for cancer drug development, and has recently published a commentary on the topic in the Journal of Pathology.
Oxford Science Blog: What is angiogenesis and why is it important in cancer growth?
Francesco Pezzella: The term angiogenesis was first recorded in 1787 and describes the formation of new blood vessels from pre-existing ones. In 1971 Judah Folkman introduced the hypothesis that tumour growth is strictly dependent on this phenomenon because, in his own words, 'Once a tumour take has occurred, every increase in tumour cell population must be preceded by an increase in new capillaries converging on the tumour'. Eventually 'inducing angiogenesis' became considered as one of the hallmarks of cancer, as it was assumed that all the tumours could only grow if they were able to induce formation of new blood vessels.
OSB: New cancer drugs have targeted the growth of new blood vessels. How have they fared?
FP: Not well! In the mid-1990s there were high hopes that these drugs would have produced a quantum leap in the treatment of all the types of tumours. The idea was that by inhibiting the formation of new vessels, all tumours would simply die or, at least, enter a quiescent state. The high expectations of the time are shown in an interview James Watson gave in 1998, when he declared that 'Judah (Folkman) is going to cure cancer in two years'.
While some benefits have indeed been observed, these have occurred only in a subset of cancers and have been modest. More worryingly, both in some animal models and human trials, progression of tumours during anti-angiogenic treatment has sometimes led to a poorer outcome being observed.
OSB: What has your research shown instead?
FP: We were as enthusiastic about angiogenesis as everybody else. However, as soon as we entered the field in 1996, our group discovered that a type of lung cancer called non small cell lung carcinoma could grow in humans without inducing any angiogenesis, and that this was also the case in advanced metastatic disease.
Our observations were in complete contrast to the accepted wisdom of the time: tumours behaving like this were just not supposed to exist.
The tumours we identified still have a blood supply, but this is obtained by exploiting, or co-opting, pre-existing blood vessels. These tumours therefore have an underlying biology which is different from that of angiogenic malignancies.
It had been accepted as a general rule that, as a tumour outgrows its blood supply, it starts to receive less oxygen and therefore pathways leading to the formation of new vessels are activated.
Instead, the tumours we described – despite having lower levels of oxygen – are not inducing any new blood vessel formation. They are responding to the diminished availability of oxygen in a different way that we still do not fully understand, but it is potentially linked to a re-programming of the way in which the cancer cells produce energy.
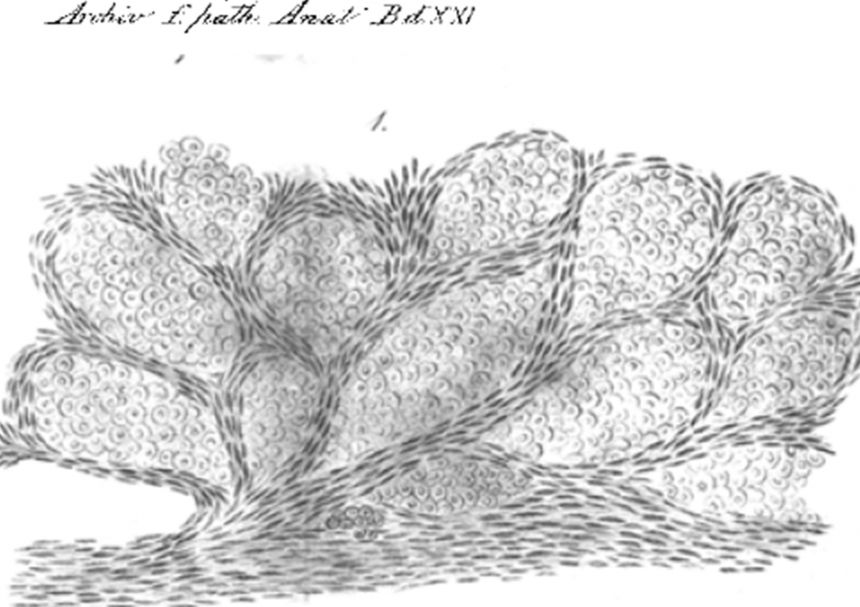 Camera lucida drawing from 1861 showing cancer cells growing inside lung alveolar spaces
Camera lucida drawing from 1861 showing cancer cells growing inside lung alveolar spacesHowever these histopathological observations have been widely ignored in favour of data from animal models, which had pointed to an absolutely essential role for angiogenesis in cancer.
OSB: Do different types of cancers, or at different stages, maintain their blood vessel supply in different ways?
FP: Yes, a primary angiogenic tumour can relapse years later as a non-angiogenic tumour, and vice versa. Even within the same tumour there can be areas attracting new vessels and areas exploiting pre-existing ones. This is actually a rather common scenario.
OSB: What are the next steps in your research/what are the open questions we now should explore?
FP: There are now two main key questions to be answered:
1) Why in some cancers is there no angiogenesis, and how can a tumour switch from angiogenic to non-angiogenic status and vice versa? Recently animal models for non-angiogenic growth have become available and they are allowing us to start to tackle the problem by mapping the pathways involved. Once we have a clearer idea of the pathways involved, we can identify the molecular mechanisms at work by trying to switch the cells from one status to another.
2) How do cancer cells co-opt blood vessels? Only a few studies have so far addressed this issue, but already we know that is a promising field because, by understanding which proteins are mediating this process, we can think about disrupting vascular co-option with drugs. This approach could deliver some useful treatments. Furthermore there are clues suggesting that the blood vessels co-opted by the tumour start to express proteins which they normally would not produce, making them different from other, normal blood vessels and therefore a possible target for treatment.
OSB: What does this all mean for new cancer treatments?
FP: Our observations explain some of the poor results observed with drugs directed only against angiogenesis. But while we have put aside forever the idea of a magic bullet able to kill all new blood vessels and cure cancer, we have also unveiled potential targets for new treatments.
We can now aim to target the pathways responsible for the metabolic re-programming of cancer cells, and any other pathways yet to be identified as responsible for the non-angiogenic behaviour. We would move from not bombing just the roads and the railways, but the factories too!
The discovery that cancer cells can survive by co-opting vessels opens an opportunity to test compounds and therapeutic antibodies able to block the co-option, and a few first candidates to target with drugs are already emerging.
Although we cannot be as optimistic as James Watson was in 1998, we think that the more complete picture we are getting of the interaction between cancer cells and blood vessels is likely to deliver some improvement in treatment in the future by targeting both angiogenesis and taking over of existing vessels.
Renowned tenor Ian Bostridge was in Oxford last week as the Humanitas Visiting Professor of Classical Music and Music Education.
To an audience of students, academics and interested members of the public, he gave a lecture entitled "Why Winterreise? Schubert's Song Cycle, Then and Now" and led a masterclass/open rehearsal and an all-day symposium.
In his lecture, Mr Bostridge discussed the meaning of a liberal education. He stressed the importance of both the arts and sciences and warned against judging the value of each by 'the methods of accountancy and the business school'. He told the audience: 'One of the main ways through which this ideal of liberal education works, in its vision of unconstrained but disciplined intellectual exchange, is through what one might call the circulation of metaphor, so that the most unlikely of disciplines can offer inspiration to each other. Sciences inspire the arts, the arts the sciences.
'My favourite historical example involves music. Musical metaphor has played a crucial role as midwife in the physical sciences from the time of Pythagoras. Musical theory was a crucial, if publicly underplayed, component in Isaac Newton’s understanding of light – through an analogy between the colour spectrum and the musical scale. The seven notes of the scale before the return to the octave are analogous to the colours of the rainbow – red, orange, yellow, green, blue and violet, plus the strangely superfluous indigo which made the number up to seven.
'More significantly, Newton interpreted Pythagoras’s views on musical consonance as containing the essence of the inverse square law of gravitation, his dazzling solution to the unity of celestial and terrestrial dynamics. Thus Newton, a sort of Pythagorean magus, reinterpreted the notion of the harmony of the spheres.'
A longer extract of this lecture can be found here.
Mr Bostridge has had a distinguished international recital career. His operatic recordings have won major international record prizes and been nominated for 13 Grammys. He was previously a fellow in history at Corpus Christi College, Oxford, and has received honorary fellowships from Corpus Christi College and St John’s College. He was made a CBE in the 2004 New Year's Honours.
This professorship was been made possible by the generous support of Mick Davis and was being hosted by Professor Jason Stanyek in association with St John's College. Humanitas is a series of Visiting Professorships at the Universities of Oxford and Cambridge intended to bring leading practitioners and scholars to both universities to address major themes in the arts, social sciences and humanities. Created by Lord Weidenfeld, the programme is managed and funded by the Institute for Strategic Dialogue with the support of a series of generous benefactors and administered by TORCH | The Oxford Research Centre in the Humanities.
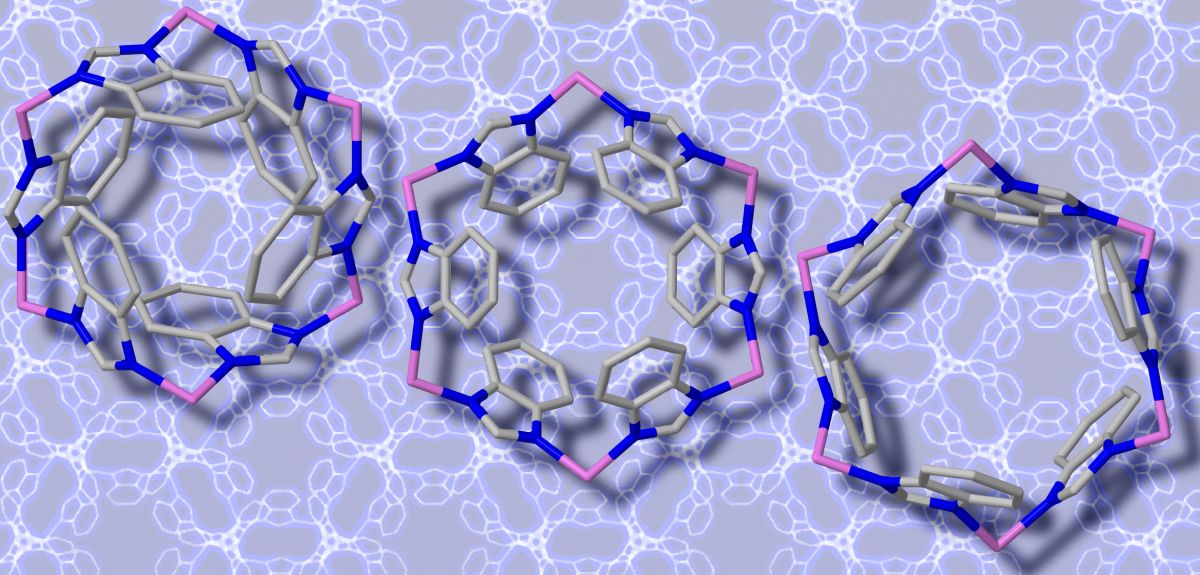
A combination of supercomputer calculations and a bombardment of high-energy particles has revealed how a new kind of material opens its pores and 'breathes'.
Metal-organic frameworks (MOFs), are formed from building blocks made up of metal ions connected by organic molecules. These molecular blocks assemble themselves to produce a variety of crystal-like structures whose porous nature and ‘shape-shifting’ abilities could make them ideal for emerging applications, such as trapping greenhouse gases or delivering drugs.
'One of the key selling points of MOFs is the exceptionally large internal surface area that some of the frameworks can possess,' Matthew Ryder, a DPhil student in the Multifunctional Materials & Composites (MMC) Laboratory at Oxford University's Department of Engineering Science, tells me. 'Some MOFs have internal surface areas as large as 10,000 square metres per gram and to put that into perspective, that's a larger surface than a football field in every gram of MOF material!'
Whilst MOFs are similar to traditional microporous materials, such as inorganic zeolites or the activated carbons used to filter drinking water or air, they typically have a surface area ten times greater and can be processed at much lower temperatures. MOFs can also be built from a wider range of metal ions and organic links so that the desirable characteristics, such as pore size and its functionality, can be 'fine-tuned'.
Unlike activated carbons, MOFs are highly crystalline and this means that their 3D crystal structure can be precisely determined using diffraction techniques, such as X-rays and neutrons. Accurate 3D representations of MOF structures are central to computational modelling studies.
'It has been suggested for some time that the practical functionalities of each specific MOF material is intrinsically controlled by its elastic responses and collective vibrations of the porous framework (called 'lattice dynamics') down at the molecular scale,' Matthew explains.
In a study recently published in Physical Review Letters the team, led by Oxford's Professor Jin-Chong Tan, reports a new method for investigating how MOFs vibrate. They tested their ideas on a subclass of MOF materials: Zeolitic Imidazolate Frameworks (ZIFs).
Their method used Density Functional Theory (DFT) to unravel the complete vibrational nature of the frameworks at the molecular level. These calculations were so demanding that they could only be accomplished on state-of-the-art supercomputers (at ARC in Oxford, SCRAF at Rutherford Appleton Laboratory in Harwell, and the SuperMUC Petascale System near Munich). The theory was then confirmed using high-resolution spectroscopic experiments at Diamond Light Source and the ISIS Pulsed Neutron & Muon Source at Harwell, Oxford.
The team found that the experiments closely matched the theoretical DFT predictions across the entire vibrational spectra and discovered that the most exciting MOF framework vibrational behaviour was located in the low-energy or 'Terahertz (THz) region'.
'We demonstrated for the first time that the Terahertz modes not only show the standard lattice vibrations, but also reveal all of the physical characteristics unique to the specific MOFs we studied (ZIF-4, ZIF-7 and ZIF-8),' Matthew tells me.
'Our results revealed intriguing Terahertz vibrational modes [watch animations here], which include co-operative 'gate-opening' and 'breathing' of the nano-sized pores of MOFs, crucial for the understanding of gas separation, storage, and sensing.
'Significantly, this study enabled us to gain new insights into mechanical properties of MOFs, elucidating possible phase change mechanisms (called 'soft modes') through which the porous framework may destabilise, distort or even collapse when subject to mechanical forces, thereby completely destroying their functionality. Furthermore, soft modes may give rise to anomalous and counter-intuitive mechanical behaviour, such as negative thermal expansion and auxeticity.'
By studying the Terahertz vibrations in MOFs the researchers believe they could pinpoint and overcome deformation mechanisms that could otherwise make them difficult to use commercially.
Understanding how MOFs vibrate, change shape, and 'breathe', could also make it possible to enhance how they trap specific gas molecules – such as greenhouse gases – and help to tailor them for the targeted delivery of anti-cancer drugs.
'Interestingly, the latest research into MOFs has concentrated on other less conventional applications of porous materials: everything from microelectronics and information storage, to water splitting for sustainable hydrogen production and solar energy harvesting (photovoltaics) for clean electricity generation,' Professor Tan comments.
'Engineers, materials scientists and chemists have a big role to play to ensure the future success of MOFs. Discovering more about the mechanical properties and long-term durability of these materials will be key to realising their full potential and making the leap from the laboratory into large-scale commercial applications.'
- ‹ previous
- 158 of 252
- next ›



 10 years on: The Oxford learning centre making an impact
10 years on: The Oxford learning centre making an impact Oxford and The Brilliant Club: inspiring the next generation of scholars
Oxford and The Brilliant Club: inspiring the next generation of scholars New course launched for the next generation of creative translators
New course launched for the next generation of creative translators The art of translation – raising the profile of languages in schools
The art of translation – raising the profile of languages in schools  Tracking resistance: Mapping the spread of drug-resistant malaria
Tracking resistance: Mapping the spread of drug-resistant malaria Cities for cycling: what is needed beyond good will and cycle paths?
Cities for cycling: what is needed beyond good will and cycle paths?