
Image credit: t.zeyrek/ Shutterstock
Fighting HIV on World AIDS Day
1st December each year marks World Aids Day: an opportunity for people worldwide to unite in the fight against HIV, and remember the over 35 million people who have died of HIV or AIDS. This death toll makes AIDS one of the most destructive pandemics in history, and an estimated 37 million people (including over 100,000 in the UK) live with the virus.
The human immunodeficiency virus (HIV) leads to acquired immunodeficiency syndrome (AIDS), a condition where the body’s immune system increasingly begins to fail, leading to life-threatening opportunistic infections and cancers. Without treatment, the average survival time after HIV infection is estimated to be 9 to 11 years. Researchers at Oxford University have led the fight against HIV/AIDS for many years, from tracking where the global spread of HIV first began, to leading trials of potential HIV cures.
The Oxford Science Blog caught up with one such researcher, Professor Lucy Dorrell, a scientist and a clinician at the Nuffield Department of Medicine, to ask how her work aims to defeat AIDS.
OxSciBlog: How has the treatment for HIV/AIDS changed over the years?
Lucy Dorrell: I started working with AIDS patients in London in the early 1990s, and back then, we had no effective treatments – all we could offer was palliative care. We had a ward with sick patients, and we tried to make the end of their lives as comfortable as possible. But a few years later, the same ward was practically empty: while we still occasionally get patients where HIV has been diagnosed at quite a late stage, when the patients are already very, very sick, HID/AIDS treatment has been completely transformed.
The main determinants of health for HIV patients are the same as for everybody else: not smoking, eating a healthy diet, exercising, etc. This is a very different message from the early years!
What brought about this transformation is the finding that the HIV virus can be completely blocked from replicating and making more copies of itself, by using a combination of antiretroviral drugs. This allows the immune system to recover, and people then don't get AIDS, even though they still have HIV in their body. It took a while to develop an effective treatment: while the basic finding was uncovered in 1996, the early drugs were really quite unpleasant: they had a lot of side-effects, and people had to take lots of pills each day.
Now, the majority of people just need to take one or two pills a day, and they will be fine – I spend a lot of time as a medical doctor telling patients that they will have a near-normal life span if they take their treatment every day. Instead, the main determinants of health for HIV patients are the same as for everybody else: not smoking, eating a healthy diet, exercising, etc. This is a very different message from the early years!
OSB: How do these drugs work?
LD: Antiretroviral drugs target the key enzymes that HIV uses to make DNA copies of its RNA and to produce the proteins that are then assembled into new viruses. Newer drugs are available that block the virus from inserting the DNA copies it has made into the host cell chromosomes.
The problem with this treatment is that people have to take it for the rest of their lives: it doesn't provide a cure. Once HIV gets into the body, it infects a subset of white blood cells, CD4 T cells, a really key part of the immune system. Once a cell is infected, it might be killed straight away, but it might also start generating new copies of HIV. But most often, HIV just goes into a cell and hides in a dormant form.
This is a big problem, because these cells, carrying HIV's genetic material, can survive in the body for a long time. If that cell is activated – say, by signals to the immune system such as contact with another infection- it will start making new copies of the virus. These new copies then go on to infect more cells, and the cycle repeats. The drugs that we currently have can't clear the body of this form of non-replicating HIV, since they work by targeting enzymes involved in HIV replication.
This dormant form of HIV is invisible to the body’s own immune system.
What is more, this dormant form of HIV is invisible to the body’s own immune system as well: the immune system detects and destroys infected cells by looking for virus-produced proteins on the cell surface. But if the virus is not replicating, it is not making any proteins.
The one silver lining is that in this dormant form, the virus is much less infectious, and therefore much less likely to be passed on. This is another part of the success story of antiretroviral drugs; they not only stop people dying, but they stop the infection being passed on to other people. This is part of the reason that number of new HIV infections is going down globally.
OSB: What is the alternative approach that your research takes?
LD: We want to find an alternative to having people take drugs for the rest of their life. We are trying to find ways to get the body's own immune system to fight HIV better, and fully remove it from the blood stream. We’re working on getting the immune system to ‘see’ infected cells better so that they can be targeted for removal. Combining this kind of immune-based strategy with a drug treatment is one way to eradicate the population of long-lived cells which are harbouring the dormant virus. If this treatment works, then we hope that one day, people infected with HIV will not have to take drugs for the rest of their lives.
What currently looks likely to work best is a combination of two different strategies: first, finding a way to reveal the dormant virus hiding in infected cells, reactivating it very carefully so that the virus then doesn’t spread to other cells. Second, to beef up the body's natural immune response, so that when infected cells are woken up, the immune system kills these cells straight away.
This strategy capitalizes on the fact that the immune system does put up a good fight against HIV, even without any treatment. It is just that HIV is a step ahead, because it is able to mutate so quickly: the body might be able to generate an effective immune response to one particular HIV protein segment, but if the virus mutates in that region, then that immune response stops being effective. This happens again and again, right from the moment HIV first gets into the body, stopping only when we give the treatment that stops HIV making more copies of itself. At this later stage, the immune system isn't very effective at fighting HIV at all.
OSB: How do you put this approach into practice in the clinic?
LD: We're working on the second strategy, of making the immune system more effective at fighting HIV.
We've been trying to understand why the natural immune response is not effective enough, and what it needs to do in order to be effective. One of the things that we have been working on is ways of defining what constitutes an effective immune response, and what we eventually want to do is make a vaccine.
There is no one in the world who, once infected, has been able to clear the virus from their body with no external treatment.
This is particularly difficult, because unlike many other diseases, there are no naturally-occurring examples of an immune system that has successfully fought off HIV completely: there is no one in the world who, once infected, has been able to clear the virus from their body with no external treatment. The only person to ever have been cured of HIV/AIDS is an American called Timothy Ray Brown, who received a bone marrow (stem cell) transplant for Leukaemia while he was HIV positive. The bone marrow donor had a two copies of a gene that confers resistance to HIV: people with both copies cannot be infected with most HIV strains, but unfortunately this is very rare – roughly 1% of people with European ancestry have this variation, and it is even rarer elsewhere.
The bone marrow transplant meant that Timothy essentially got a new immune system – one which couldn't be infected with HIV!
Bone marrow transplantation carries a lot of risks, so this can't be the standard treatment. But people are beginning to use personalized genetic engineering to do something similar.
The problem with this approach is that it is difficult to scale it up to treat millions of people, and the fact that there are no naturally occurring examples of someone successfully clearing HIV from their body means we can't learn from nature when it comes to AIDS. So we're having to approach this in a completely different way.
OSB: Are there natural variations in HIV immune responses that you can exploit?
There are very rare (less than 1% of the HIV-infected population) individuals who are called long-term non-progressors: they have undetectable levels of the virus in their blood, so their diagnostics look like they are being treated with antiretroviral drugs, even when they have had no treatment at all.
LD: There are very rare (less than 1% of the HIV-infected population) individuals who are called long-term non-progressors: they have undetectable levels of the virus in their blood, so that their diagnostics actually look like they are being treated with antiretroviral drugs, even when they have had no treatment at all! These individuals remain healthy and somehow seem to be able to keep the virus at a very low level for a long period of time, without any treatment.
We and many other groups have been studying this group of patients for a long time, to try and work out what is different about their immune response. We’ve learnt that a lot of this naturally-occurring resistance has to do with the genes coding parts of the immune response to virus-infected cells. But that is not the whole story, and in our lab, we've been studying how specialized immune cells from these patients deal with replicating HIV in a culture. We've developed a test that mimics, as much as possible, what we think is going on in a real immune response in the body.
What this line of work has shown is that long-term non-progressors are at one end of a spectrum, and while it seemed that they may be in a special class all by themselves, there are others who share some of their characteristics but to a lesser extent. There was a feeling that this was really the only group of patients worth studying, but we've shown, by carefully measuring immune responses from a variety of patients in the lab, that an effective immune response is not an all-or-nothing property.
The big question now is whether we can nudge someone on one end of the spectrum of immune responses to the other, so that they are able to fight of the virus better.
OSB: How are some people able to fight HIV better?
LD: Looking at the naturally occurring variation in immune system responses, it is clear that what happens when the immune system first confronts the virus is very important, so there is a real push to give treatment for HIV as early as possible.
The second key factor to the success of the immune response seems to be where it is targeted: the HIV genome is unstable and changes all the time, which means that the virus can afford to take a lot of hits from the immune system, since their target simply mutates without doing much damage to HIV. But there are part of the HIV genome where the virus cannot afford this strategy, and hitting these more conserved regions really does make it more likely that the virus will be killed.
People who can mount a better immune response against HIV generate more hits against the virus, which makes sense: the more hits the virus takes, the greater the chances that one of them will land in one of these vulnerable regions. But rather than having a scattergun approach, these more effective immune responses also seem to be able to generate more well-targeted hits against vulnerable HIV regions.
OSB: How are you trying to nudge the immune system to be more effective?
LD: We're trying to leverage these naturally-occuring strategies to produce a vaccine for people already infected with HIV: we've been carrying out clinical trials with HIV positive patients over the last ten years, and our current trial is on patients who have been given treatment within weeks of being exposed to the virus. We vaccinated 24 of these patients with a vaccine that we've developed in Oxford – the vaccine tries to hit the virus in its most vulnerable parts, where it is least likely to mutate.
This is going to be a long, step-by-step process and we're likely to see small changes to start with, but we can then try and come up with ways of making these into bigger changes!
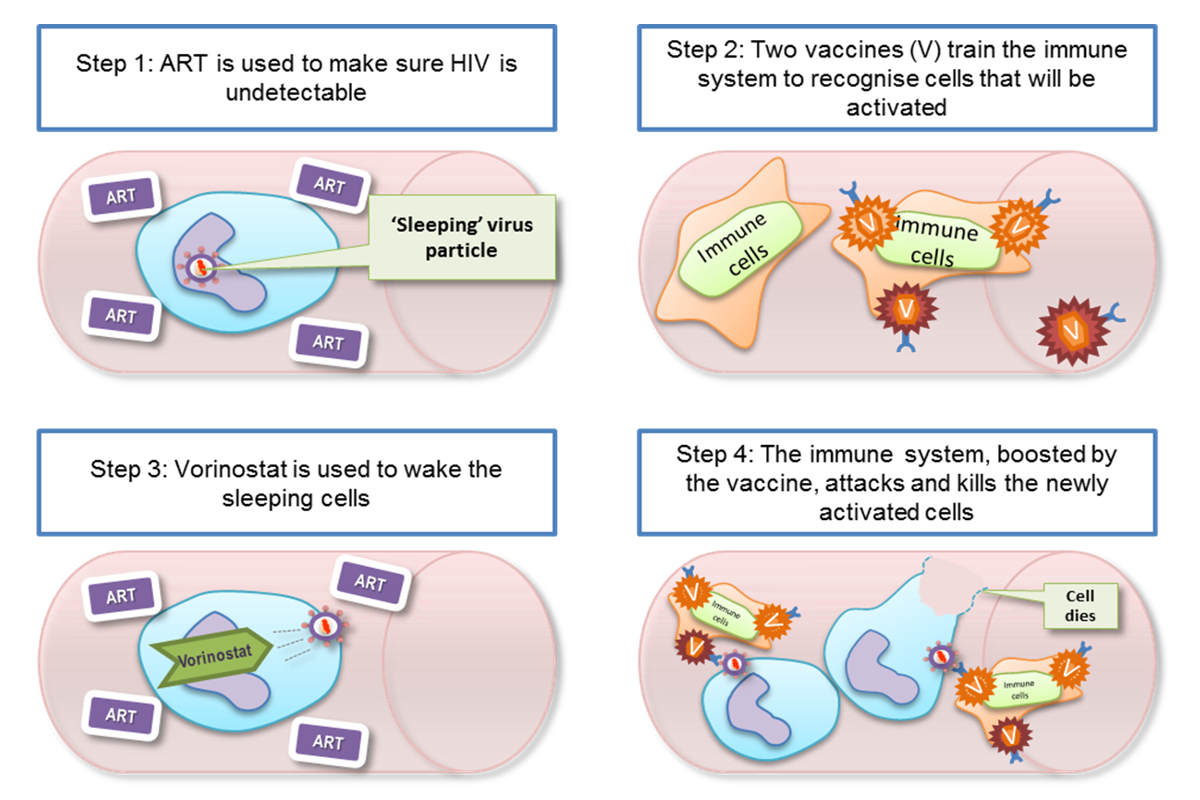
We’re currently looking at what is happening in these patients' immune systems as a result of this beefing up. We're also tracking what happens to the virus reservoir: the total number of infected cells where the virus is hiding. We're interested in seeing whether this reservoir will change over time – it did not change much with just the antiretroviral therapy combined with vaccination. So the next step will be to actually 'wake up' the virus, and then see what happens to it when this unmasking is combined with the vaccination and the antiretroviral therapy.
We hope that the vaccination will produce plenty of specially primed killer T cells that are ready and waiting to eliminate those long-lived CD4 T cells carrying dormant virus as soon as they are 're-awoken'. If this goes according to plan, then we should see a reduction in the HIV reservoir in the body.
I think this is going to be a long, step-by-step process and we're likely to see small changes to start with, but we can then try and come up with ways of making these into bigger changes!