Mathematical models developed for the oil industry may transform the way we bring stroke drugs to market
By Dr Wahbi El-Bouri
There are over 1.2 million stroke survivors in the UK, with 100,000 strokes happening in the UK each year. That’s the equivalent of one stroke every five minutes. They are also the leading cause of disability in the Western world.
Research underway in the Department of Engineering Science’s Cerebral Haemodynamics Group, headed up by Professor Stephen Payne, is changing our understanding of blood flow around the brain. Here, I explain how this could speed up the arduous process of bringing stroke drugs to market.
Our research has two main questions. Firstly, can we model blood and oxygen transport in the entire human brain, across the billions of blood vessels present? And secondly, can we run in-silico clinical trials (that is, trials performed entirely on a computer) of stroke and stroke treatment?
This is what we are tackling as part of a Horizon 2020 project (In-Silico Trials for Treatment of Acute Ischaemic Stroke) alongside European research collaborators from 10 other institutes, including radiotherapists, clinicians, academics, and industrial partners.
A continuous supply of oxygen and glucose, via the bloodstream, is essential to maintain healthy brain function under all circumstances. Whereas the rest of our body can release stored-up energy when we feel hungry, the brain has no such reserves. As such, any reduction in blood flow to the brain, even if only for a few minutes, can lead to cell death and loss of brain function. A large reduction of blood flow for a prolonged period of time, whether through a blocked or ruptured vessel, is known as a stroke.
In addition, dementia (the leading cause of death in the UK) is increasingly being linked to changes in our smallest blood vessels, or microvasculature, as we age. There is clearly an urgent need to understand the mechanisms of stroke and brain ageing in order to combat these debilitating diseases.
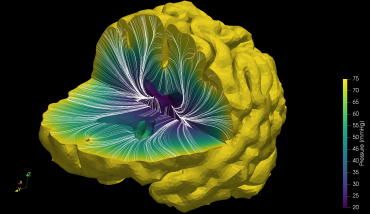 Capiliary velocity
Capiliary velocityThe starting point for these models must be real-life data. Unfortunately, due to the low resolution of current clinical imaging modalities, the only way we can currently ‘see’ the smallest vessels in the brain (which average 1/10th the width of a human hair) is to image slices of dead human brains. Using these, researchers construct networks on a computer and simulate blood and oxygen transport. However, we quickly run into the problem of scaling up these networks to encompass the billions of blood vessels that make up the human brain.
Our team is using mathematical tools developed for use in the oil and gas industry, who have been trying to model water and oil flow through rock for decades. We treat the brain as a chunk of porous rock and hence approximate the flow through our brain, as opposed to modelling the flow in each individual vessel. This allows us to rapidly produce full brain computer models of blood and oxygen transport!
The models that we are developing, along with models of clot formation, clot removal using a stent and thrombolysis (dissolving the clot with drugs), will be used to run clinical trials on computers that can be personalised and help to inform real-life clinical trials. For example, certain geometries of blood vessels or certain clot positions may be more amenable to a certain treatment.
This knowledge, from the in-silico clinical trial, can then inform a real-life trial to target treatment to those people and hence improve the chances of that stroke treatment being brought to market and used to save lives. In the future, these models could be used to simulate a variety of neurological diseases and help us to understand the human brain, in both health and disease.
Read more about the Cerebral Haemodynamics Group’s research.
Find out more about Brain Awareness Week at Oxford.
This article was published to mark Brain Awareness Week, a global campaign running from 11-17 March.