Study shows diverse gut bacteria communities protect against harmful pathogens by nutrient blocking
A new study led by the University of Oxford has demonstrated that diverse communities of resident bacteria can protect the human gut from disease-causing microorganisms. The researchers found that protective communities block the growth of harmful pathogens by consuming nutrients that the pathogen needs. The findings, published today in the journal Science, could help to develop new strategies to optimise gut health.
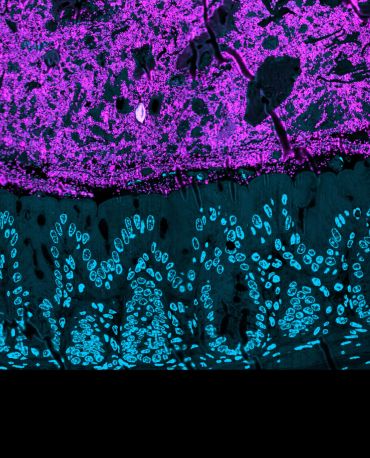 Bacteria cells in the mammalian gut. The bacteria cells (magenta) are sitting above the cells of the gut wall (nuclei shown as blue circles). Image credit: Martin Jahn.
Bacteria cells in the mammalian gut. The bacteria cells (magenta) are sitting above the cells of the gut wall (nuclei shown as blue circles). Image credit: Martin Jahn.To investigate this, researchers at the University of Oxford tested 100 different gut bacteria strains individually and in combination for their ability to limit the growth of two harmful bacterial pathogens: Klebsiella pneumoniae and Salmonella enterica. Individual gut bacteria showed a very poor ability to restrict the spread of either pathogen. But when communities of up to 50 species were cultured together, the pathogens grew up to 1000 times less effectively than when cultured with any individual species. This ‘community protection effect’ was seen regardless of whether the bacteria were cultured together in vials, or in ‘germ-free’ mice (which had no resident gut bacteria at the start of the experiments).
Author Professor Kevin Foster (Departments of Biology and Biochemistry, University of Oxford) said: ‘These results clearly demonstrate that colonization resistance is a collective property of microbiome communities; in other words, a single strain is protective only when in combination with others.’
However, the researchers found that the members of the bacterial communities – and not just the overall diversity – had a critical effect on the level of protection. Certain species were found to be essential for community-based protection, even though these species offered little protection on their own.
The researchers demonstrated that protective bacterial communities block pathogen growth by consuming the nutrients that the pathogen needs. By assessing the genomes of the different bacterial species, they found that the most protective communities were composed of species with highly similar protein compositions to the pathogenic species. They also used metabolic profiling to demonstrate that the protective species had similar demands for carbon sources as the pathogens.
Author Frances Spragge (Departments of Biology and Biochemistry, University of Oxford) added: ‘Crucially, although increased microbiome diversity increases the probability of protection against these pathogens, the overlap in nutrient utilization profiles between the community and the pathogen is key. Certain species that have a crucial role in community protection show a high degree of metabolic overlap with the pathogen, and therefore similar nutrient demands.’
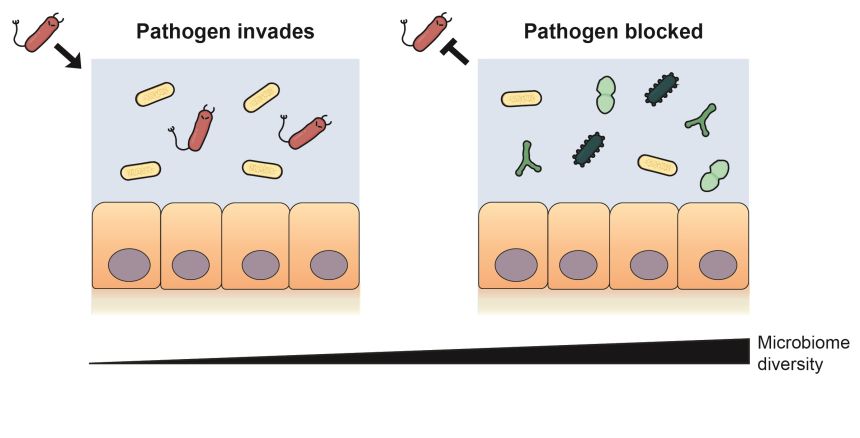 Nutrient blocking: diverse gut bacteria consume the nutrients that a pathogen needs to invade and block it from the microbiome. Credit: Erik Bakkeren
Nutrient blocking: diverse gut bacteria consume the nutrients that a pathogen needs to invade and block it from the microbiome. Credit: Erik BakkerenThe researchers used this nutrient blocking principle to predict communities of bacteria that would offer weak and strong protection against a different pathogen: an antimicrobial resistant E. coli strain. When tested experimentally, the communities which had the highest nutrient overlap with the E. coli strain were up to 100-fold more effective at reducing the pathogen’s abundance than the communities predicted to give weak protection.
According to the researchers, these new insights could be developed into novel strategies to combat harmful gut pathogens through optimising gut microbiome communities. They may also explain why individuals can become more susceptible to species such as K. pneumoniae after taking antibiotic treatments that can lower the diversity of gut microbiome species.
Author Dr Erik Bakkeren (Departments of Biology and Biochemistry, University of Oxford) added: ‘Our work supports the general hypothesis that a more diverse microbiome can carry health benefits. This gives promise to the goal of optimising the composition of microbiomes to protect against bacterial species that are harmful to health.’
The study ‘Microbiome diversity protects against pathogens by nutrient blocking’ has been published in Science.
 Largest ever UK study reveals stark ethnic and social inequalities in lung cancer diagnosis
Largest ever UK study reveals stark ethnic and social inequalities in lung cancer diagnosis
 Oxford’s gargoyles come to life in new Extended Reality (XR) interactive experience
Oxford’s gargoyles come to life in new Extended Reality (XR) interactive experience
 Treating bullying as everyone’s problem reduces incidence in primary schools
Treating bullying as everyone’s problem reduces incidence in primary schools
 Oxford's Ashmolean Museum saves Fra Angelico masterpiece to go on public display from December
Oxford's Ashmolean Museum saves Fra Angelico masterpiece to go on public display from December
 New, ARIA-backed project aims to unlock radically cheaper AI hardware
New, ARIA-backed project aims to unlock radically cheaper AI hardware
 First evidence drug resistant bacteria can travel from gut to lung, increasing infection risks
First evidence drug resistant bacteria can travel from gut to lung, increasing infection risks
 Gut bacteria linked to personality
Gut bacteria linked to personality