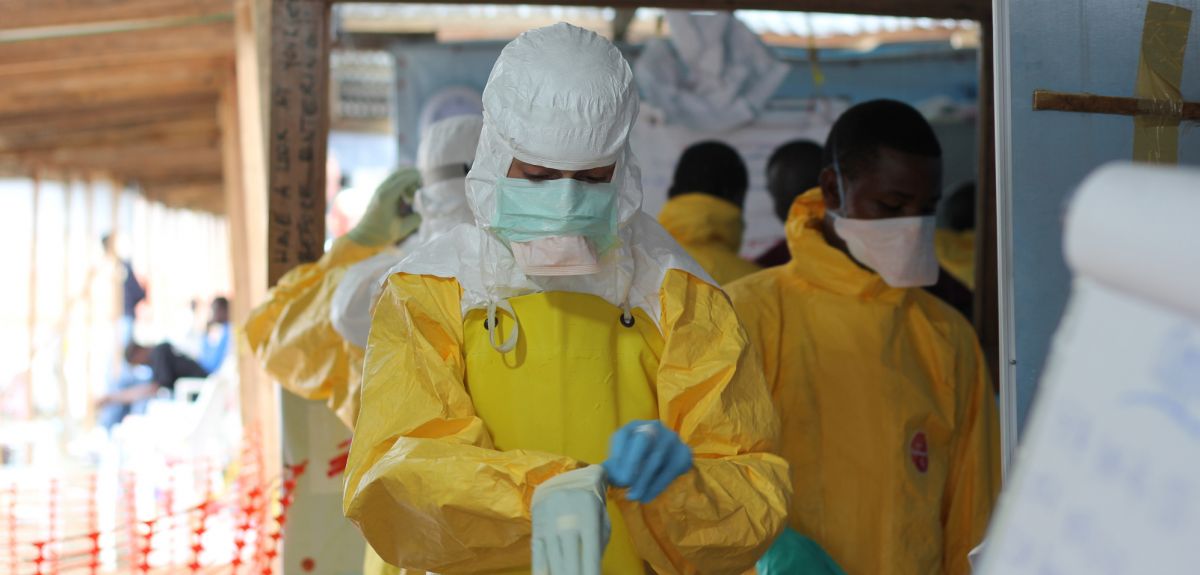
Image © Caitlin Ryan/MSF
Oxford to lead trial of experimental drug in Ebola patients
A clinical trial is to be carried out in West Africa to see whether a novel antiviral drug called brincidofovir is effective against Ebola, subject to regulatory approval.
The trial, involving an international team led by Oxford University scientists, will take place in an Ebola treatment centre run by Médecins Sans Frontières (MSF).
Brincidofovir is an experimental antiviral drug made by Chimerix of Durham in North Carolina, USA.
The trial is being funded by the Wellcome Trust and will be carried out by an international team, including the University of Oxford, the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC), MSF, the World Health Organisation (WHO), Institut Pasteur, Institut Pasteur de Dakar, Fondation Mérieux and the Global Health Network.
The trial protocol has been submitted to ethics committees in Oxford and West Africa, and at MSF and the WHO. It is hoped that the trial will be able to start in as soon as a few weeks' time.
Professor Peter Horby of Oxford University and ISARIC, the chief investigator of the trial, said: 'Conducting clinical trials of investigational drugs in the midst of a humanitarian crisis is a new experience for all of us, but we are determined not to fail the people of West Africa. It has been a privilege to witness the extraordinary willingness of all the partners in this initiative to step outside their comfort zones in order to fast track these critically important trials.'
The trial is one of three clinical trials of potential treatments for Ebola announced today which will be carried out by European teams at different MSF treatment centres in West Africa.
Conducting clinical trials of investigational drugs in the midst of a humanitarian crisis is a new experience for all of us
Professor Peter Horby
The French National Institute of Health and Medical Research (INSERM) will lead a trial of a drug called favipiravir (Avigan), while the Institute of Tropical Medicine in Antwerp, Belgium, will carry out a trial to see whether antibodies in the blood of Ebola survivors can help patients fight off the disease.
The principal investigators of the three trials are working closely together and with MSF and WHO to ensure that the studies are coordinated, complimentary and comparable. The results of the trials will be shared widely as soon as they are available.
'This is an unprecedented international partnership which represents hope for patients to finally get a real treatment against a disease that today kills between 50 and 80% of those infected,' said Dr Annick Antierens, who coordinates the investigational partnerships for MSF. 'As one of the principal providers of medical care to Ebola patients in West Africa, MSF is taking part in these accelerated clinical trials to give people affected by the current outbreak a better chance of survival.'
Professor Trudie Lang of Oxford University, and a member of the research team, says: 'As the Ebola crisis deepens, the problem we have is that we do not know whether any of the drugs in development would work, or would be safe to use. Could any of them help in the current epidemic? We don't know, but we need to find out as quickly as possible. The only way to determine that is through a clinical trial.
'We are working in full partnership with international organizations and colleagues in West Africa to conduct a clinical trial to find out. Typically it takes over 18 months to set up a trial, and that is in a ‘normal’ situation. Here we need to set up a trial within weeks.'
Brincidofovir has been chosen for this trial for a number of reasons. There is good data on its safety in humans from trials for other viral infections. It is easy to give: it is an oral drug, taken twice a week. And should it prove to be an effective treatment, its manufacture would be relatively easy to scale-up and make many more doses available.
Brincidofovir works by interfering with a virus' ability to replicate its genetic material. It is currently in advanced clinical trials for treating adenovirus and cytomegalovirus infections, providing a lot of data on its safety profile. Test-tube studies have shown good activity against the Ebola virus, but no studies in animal models have been done as yet.
In the trial – a single-arm, open-label study – brincidofovir would be given to all patients in the treatment centre consenting to take part.
The main outcome measure would be fatality rate among the Ebola patients taking part. This would be compared against the previous death rate seen among patients in the treatment centre before the trial began.
Up to 140 adults will be involved in the trial. The two-week treatment course would see patients with Ebola disease have two tablets on day zero, then single dose on days 3, 7, 10 and 14.
Participating patients would also be monitored and have blood samples taken. Pregnant women are the only group to be excluded from the trial, as the drug has shown some adverse effects on embryos in animal studies.
The study could also make use of an adaptive trial design, where the design can be modified as the trial progresses and data builds up. The trial can be stopped early if the drug shows clear benefits or harm. It may also be possible to add other novel drugs into the trial as they reach a point where they can be tested, or to allow comparisons between candidate drugs.
Piero Olliaro, a visiting professor at the University of Oxford and a Senior Research Manager at the WHO-based Special Programme for Research and Training in Tropical Diseases, says: 'This trial design is no short cut, no compromise on science or ethics. Non-comparative trials like this have always been done. And there are examples of non-comparative phase 2 trials in multiple forms of cancer and in viral infections.
'At the same time, a trial like this has never been done in a humanitarian disaster before. It needs innovative approaches for triaging patients and assessing their condition, all in difficult conditions in an emergency situation.
He adds: 'I strongly believe that no individual measure will solve the problem. We shouldn’t look to new vaccines or drugs as being the sole thing that will bring the Ebola epidemic under control. We need to look at the overall picture and work on all aspects: new vaccines and treatments, yes, but also containment measures and strengthening the support available on the ground.
'If brincidofovir is found to be safe and effective, there is potential for it to make a difference, for manufacture to be scaled up and for it to be available. That might not be true of all drugs that could be tested at this time.
'Even if we don't end up with a very effective drug, having something that has some efficacy means we can treat people, they might come to treatment centres earlier and they might stop transmitting the disease earlier. All of which would help in bringing the outbreak under control.'
Dr Jeremy Farrar, Director of the Wellcome Trust which is funding the trial, said: 'We are delighted to support Professor Horby and work alongside governments of West Africa and MSF whose response to the outbreak has been simply heroic. It is a huge challenge to carry out clinical trials under such difficult conditions, but this is the only way we will find out whether potential Ebola treatments work. While a therapy may not directly reduce community transmission, it would have immense humanitarian value in saving lives and reducing in-hospital transmission. It would also encourage people to seek medical care, which would make a very real difference in this outbreak.'
 Statins do not cause the majority of side effects listed in package leaflets
Statins do not cause the majority of side effects listed in package leaflets
 Activism proves a stimulating topic at Sheldonian Series event
Activism proves a stimulating topic at Sheldonian Series event
 New Oxford-led initiative launches to train future leaders in transformative technologies for pharmaceutical research
New Oxford-led initiative launches to train future leaders in transformative technologies for pharmaceutical research
 First volunteer receives new Ebola vaccine in UK trial
First volunteer receives new Ebola vaccine in UK trial
 Trials of novel Ebola drugs to be fast-tracked in West Africa
Trials of novel Ebola drugs to be fast-tracked in West Africa
 Risk of Ebola emergence mapped
Risk of Ebola emergence mapped