New laptop program can identify drug resistance from bacterial genomes
Scientists have developed an easy-to-use computer program that can quickly analyse bacterial DNA from a patient's infection and predict which antibiotics will work, and which will fail due to drug resistance. The software is currently being trialled in three UK hospitals to see whether it could help speed up diagnosis of drug-resistant infections and enable doctors to better target the prescription of antibiotics.
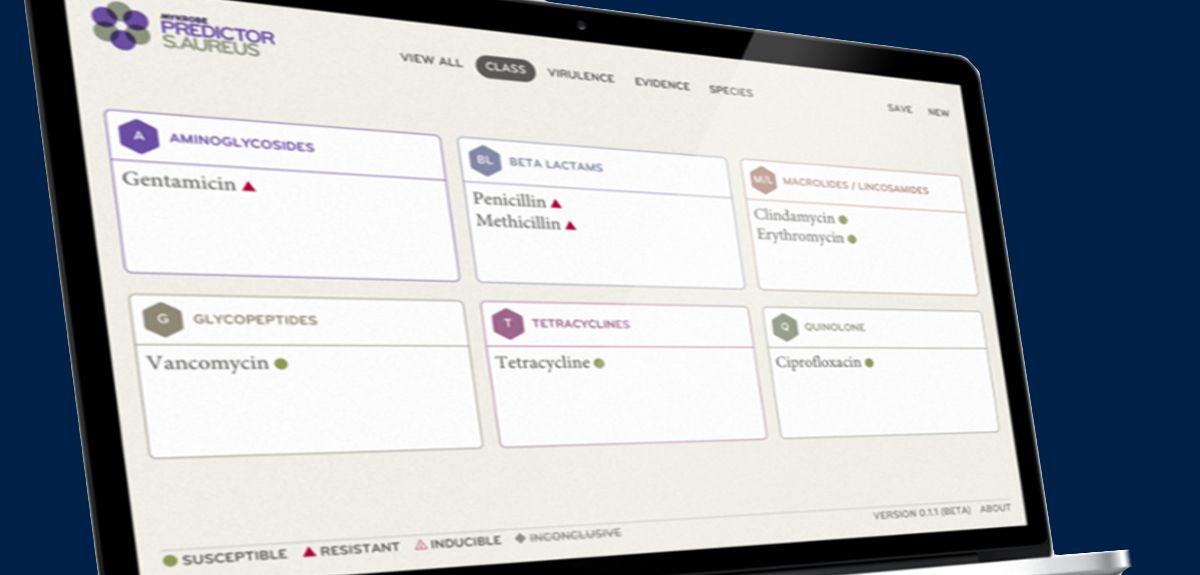
The Mykrobe Predictor software, developed by Dr Zamin Iqbal and colleagues at the Wellcome Trust Centre for Human Genetics, University of Oxford, runs on a standard laptop or tablet without the need for any specialist expertise. The program can analyse the entire genetic code of a bacterium in under 3 minutes, once a bacterial sample has been cultured and its DNA sequenced.
One of the barriers to making whole genome sequencing a routine part of NHS care is the need for powerful computers and expertise to interpret the masses of complex data. Our software manages data quickly and presents the results to doctors and nurses in ways that are easy to understand, so they can instinctively use them to make better treatment decisions.
Dr Zamin Iqbal, Wellcome Trust Centre for Human Genetics
A study on more than 4,500 retrospective patient samples, published today in Nature Communications, shows that Mykrobe Predictor accurately detects antibiotic resistance in two life-threatening bacterial infections: Staphylococcus aureus (one form of which causes MRSA) and tuberculosis (TB).
The software is now being evaluated in hospitals in Oxford, Brighton and Leeds in a project led by Professor Derrick Crook, which in collaboration with parallel programmes at UCL and Cambridge University aims to develop whole genome sequencing as a routine tool for the diagnosis and control of infections within the NHS. The programme is funded by the Department of Health and the Wellcome Trust through the joint Health Innovation Challenge Fund.
Drug-resistant infections pose a major threat to global health and could in future mean that serious and life-threatening infections become impossible to treat. The problem develops through natural selection. Like other species, bacteria are constantly evolving through subtle changes in their DNA. Some of these changes make them resistant to certain drugs, meaning they are more likely to survive and pass on their resistant traits to other bacteria.
One of the best ways to prevent the spread of resistant bacteria is to make sure that patients with an infection are treated quickly with the right type of antibiotic. But to find out which particular strain of bacteria is causing a patient's infection and which drugs it is resistant to, doctors must carry out drug susceptibility testing, where different antibiotics are applied to the bacteria in a petri dish to see whether they kill it. This process can take days, or even months for slow-growing infections like TB.
Scientists believe they could obtain the same information much faster by looking directly at the DNA sequence of the bacterium for mutations that are known to cause resistance. However, the interpretation of genetic information often requires a large amount of computing power and the expertise of specialist bioinformaticians.
Mykrobe Predictor streamlines this process by automating genome analysis, cross-checking the bacterium's DNA sequence with previous strains to look for resistance-causing mutations and presenting information about the bug in an easy-to-understand format.
In this study, the software was able to detect resistance to the five first-line antibiotics in over 99% of Staphylococcus aureus cases, matching the performance of traditional drug sensitivity testing. For TB, where the genetic basis for drug resistance is less well understood, Mykrobe Predictor still matched the performance of current DNA tests (which look at snippets of DNA, but not the whole sequence), detecting 82.6% of resistant infections around 5-16 weeks faster than traditional drug susceptibility testing. However unlike standard 'snippet-based' DNA tests, Mykrobe Predictor can be rapidly updated with a simple software upgrade that allows researchers to detect new resistance mutations as they evolve.
A further advantage of Mykrobe is that it can identify infections where a patient's body contains a mixture of both drug-resistant and drug-susceptible bacteria. In this study, the ability to distinguish between these bacterial 'sub-populations' gave Mykrobe an advantage over conventional testing in detecting resistance to second-line TB drugs. This is important for diagnosing infections such as 'extensively drug-resistant TB' (XDR-TB), which is resistant to at least four of the core TB drugs and is considered a global threat to public health by the World Health Organisation.
Dr Zamin Iqbal, from the Wellcome Trust Centre for Human Genetics at the University of Oxford, senior author of the paper, said: 'One of the barriers to making whole genome sequencing a routine part of NHS care is the need for powerful computers and expertise to interpret the masses of complex data. Our software manages data quickly and presents the results to doctors and nurses in ways that are easy to understand, so they can instinctively use them to make better treatment decisions.'
Co-author Professor Derrick Crook, a Consultant Microbiologist at the John Radcliffe Hospital and Director of the National Infection Service, Public Health England, said: 'Genome sequencing has the potential to transform the way we diagnose and treat bacterial infections in NHS hospitals, but one of the main challenges is developing the right tools to enable us to unlock this information quickly and affordably. Our ultimate goal is to be able to provide complete information on a pathogen within 24 hours of culture, linking this information to a national surveillance database to enable more timely and better targeted patient treatment.'
Dr Stephen Caddick, Director of Innovations at the Wellcome Trust, added: 'Drug-resistant infections pose a major threat to global public health. Antibiotics that were once lifesavers are in danger of becoming worthless, and within our lifetime we could see minor infections returning as a major public health concern. We urgently need new diagnostic strategies that allow us to better target antibiotic use, and thereby safeguard the effectiveness of our existing antibiotics, and any new drugs that are developed in future.'
The paper, Rapid antibiotic resistance predictions from genome sequence data for S. aureus and M Tuberculosis, is published by Nature Communications (doi:10.1038/NCOMMS10063).
 New study estimates NHS England spends 3% of its primary and secondary care budget on the health impacts of temperature
New study estimates NHS England spends 3% of its primary and secondary care budget on the health impacts of temperature
 International collaboration launches largest-ever therapeutics trial for patients hospitalised with dengue
International collaboration launches largest-ever therapeutics trial for patients hospitalised with dengue
 Oxford-built multi-agent assistant for cancer care to be piloted in collaboration with Microsoft
Oxford-built multi-agent assistant for cancer care to be piloted in collaboration with Microsoft
 World's first Phase II Nipah virus vaccine trial launch
World's first Phase II Nipah virus vaccine trial launch