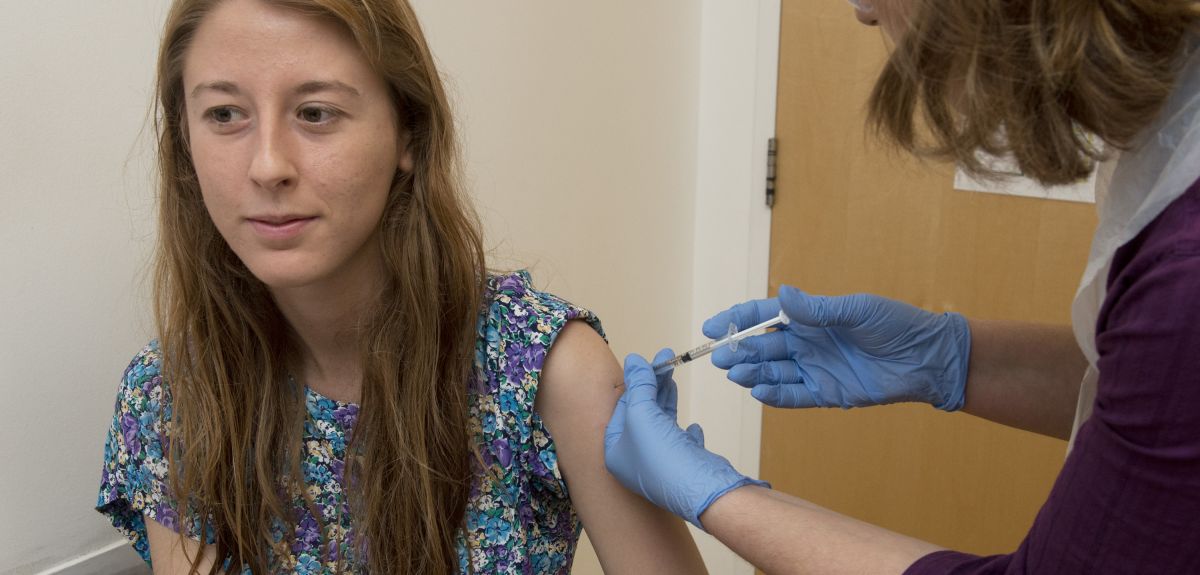
Credit: Rob Judges/Oxford University Images
Trials of an experimental Ebola vaccine progress at speed
Almost 200 people have received a candidate Ebola vaccine in little more than two months in safety trials carried out in the USA, UK, Mali and Switzerland.
The total includes:
• 20 people at the US National Institutes of Health in Bethesda, Maryland;
• 80 people at the University of Maryland School of Medicine Center for Vaccine Development in Mali (a joint venture with the Ministry of Health of Mali); and
• 34 people out of an eventual 120 volunteers will have been vaccinated at the University Hospital of Lausanne by 17 Nov.
In Oxford, the 60th and last healthy volunteer in a trial carried out by the University of Oxford is due to receive the vaccine tomorrow, on Tue 18 Nov.
The Ebola vaccine was developed by the US National Institutes of Health (NIH) and GSK. These first trials in humans have been fast-tracked in response to the current Ebola outbreak.
If the safety and immunogenicity data from the Phase 1 trials are promising, the expectation is that the vaccine will move into the next phases of study to further evaluate safety as well as effectiveness in protecting against Ebola infection in African countries.
'The safety data here have looked very satisfactory so far,' says Professor Adrian Hill of the Jenner Institute at Oxford University, who is leading the Oxford trial. 'The response we have seen from people coming forward to take part has been remarkable.'
The candidate Ebola vaccine is being co-developed by the US National Institutes of Health (NIH) and GSK against the Zaire species of Ebola, which is the one circulating in West Africa.
It uses a single Ebola virus gene in a chimpanzee adenovirus to generate an immune response. As it does not contain infectious Ebola virus material, it cannot cause a person who is vaccinated to become infected with Ebola.
Pre-clinical research by the NIH and Okairos, a biotechnology company acquired last year by GSK, has indicated that the vaccine protects non-human primates exposed to Ebola without significant adverse effects.
The first volunteer in the UK trial at Oxford University was vaccinated on 17 September, two weeks after the first volunteer in the USA. This allowed further trials in Mali and then Switzerland to begin shortly afterwards in October.
The trialists in Lausanne hope to have a total of 120 subjects vaccinated by beginning of December.
The clinical trial in Mali, being performed by the Center for Vaccine Development of Mali (CVD-Mali), the CVD of the University of Maryland School of Medicine and the Malian Ministry of Health, is providing the only clinical and immune response data so far with the vaccine in West African subjects. By late December 2014, the team should know how the immune responses of Malian health care workers given the vaccine compare to those observed in adults given the vaccine in the UK and Switzerland.
'This research is a testament to the hard work and cooperation of all the institutions involved,' says Professor Myron M Levine, director of the Center for Vaccine Development. 'If this vaccine is proven to work, it could help alter the dynamic of this epidemic by interrupting transmission to the health care workers who are most at risk.'
The Oxford University trial is being funded under a £2.8 million grant from the Wellcome Trust, the Medical Research Council (MRC) and the UK Department for International Development (DFID). The consortium's funding is also enabling GSK to begin manufacturing thousands of additional doses of the vaccine so that if Phase 1 trials are successful, the next phases of the clinical trial programme can begin – which will involve the vaccination of frontline healthcare workers in Ebola affected countries.
The NIH is providing the NIAID/GSK Ebola vaccine for the Oxford study.
 New study finds that ChatGPT amplifies global inequalities
New study finds that ChatGPT amplifies global inequalities
 Expert Comment: Chatbot-driven sexual abuse? The Grok case is just the tip of the iceberg
Expert Comment: Chatbot-driven sexual abuse? The Grok case is just the tip of the iceberg
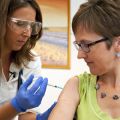 First volunteer receives new Ebola vaccine in UK trial
First volunteer receives new Ebola vaccine in UK trial
 Oxford to lead trial of experimental drug in Ebola patients
Oxford to lead trial of experimental drug in Ebola patients
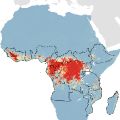 Risk of Ebola emergence mapped
Risk of Ebola emergence mapped